How do Pharmaceutical Isolators work?
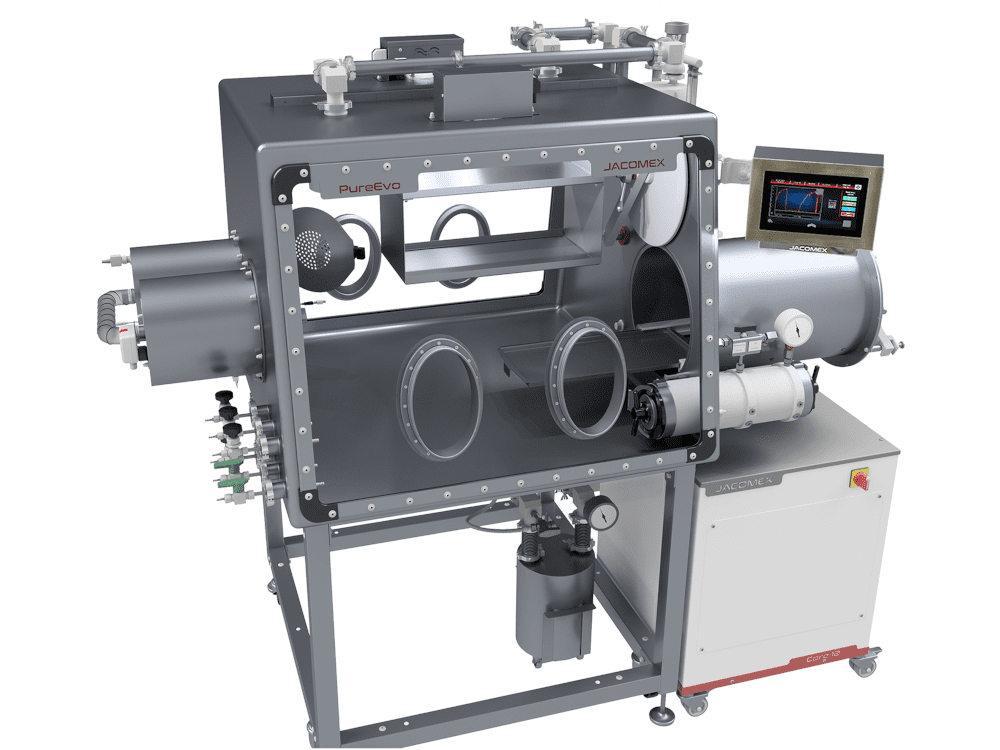
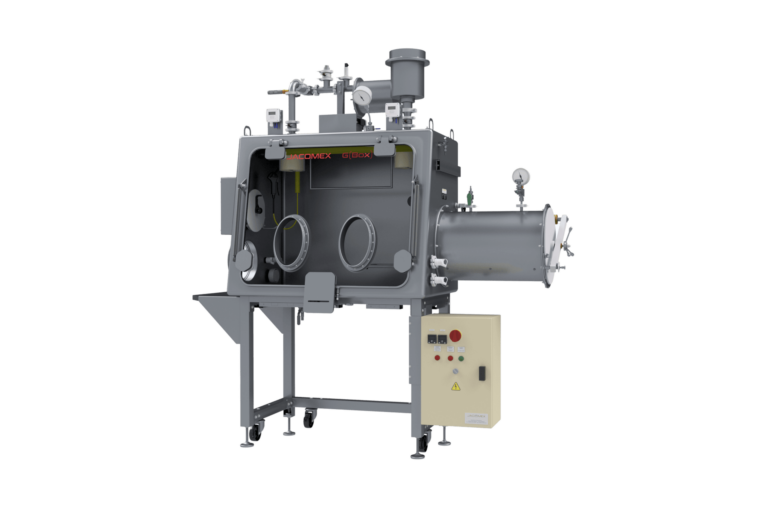
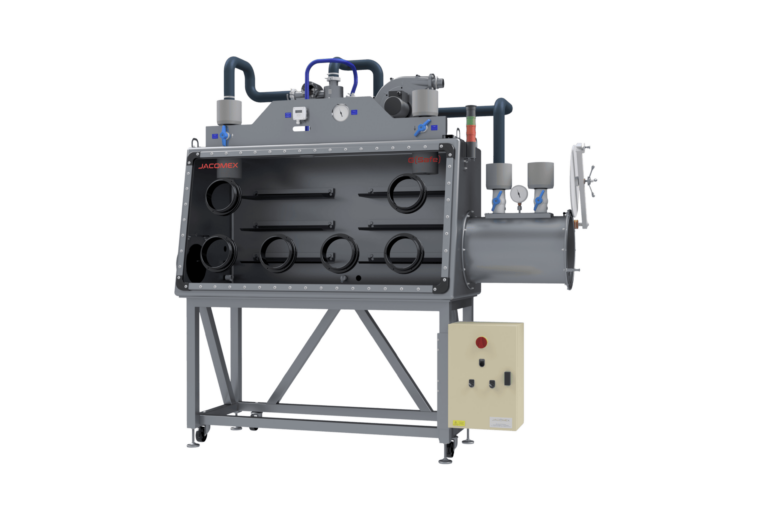
The pharmaceutical isolator is a bacteriologically sealed enclosure used in the medical and pharmaceutical environment for toxic processes and aseptic filling process. It is made of a perfectly sterile main isolator where the products are handled, stored or packaged using shoulder-high gloves placed on one of the walls. It also includes transfer systems that allow the entry and exit of the products and the elimination of waste without breaking aseptic conditions.
Essential architecture and mechanisms
The isolator is a bacteriologically sealed containment space used in the medical and pharmaceutical industries during toxic processes and aseptic distribution. It consists of a perfectly sterile main isolator where products are handled, stored or packaged using gloves that extend to the shoulders and are placed on one of the walls. It also incorporates transfer systems that allow products to be brought in and out and waste to be removed while maintaining containment and aseptic conditions.
The materials chosen for construction are non-contaminating, easy to clean and compatible with regular decontamination cycles. The surfaces are smooth, with no blind spots, to facilitate sterilisation.
The pharma isolator was designed to address two key challenges:
- Containment.
In production facilities, an isolator enables the containment of pharmaceutical processes. These processes require a protected environment, free from viable microorganisms. The isolator ensures the separation of the production area from personnel/environment. Its role is to prevent contamination from spreading from one of these areas to the other. This technology achieves a higher level of containment than traditional clean rooms. It uses an integrated decontamination system.
In general, the isolator is decontaminated using a sterilising gas: hydrogen peroxide or H2O2. It uses very high efficiency (VHE) filters for maximum product protection. These systems aim to eliminate microbes and particles from all possible sources of contamination, such as the external environment and personnel.
The decontamination cycle is fully automatable and recordable, ensuring the traceability required by pharmaceutical standards.
- Product transfer.
When introducing or removing products from the enclosure, containment and preservation of the atmosphere must be guaranteed. The transfer lock ensures this safety. This system simplifies the transit of products and materials, as well as the removal of waste. This ensures that the environment remains sterile at all times. Isolators for the pharmaceutical industry must comply with Class A standards according to the GMP EU JO 07/01/97 classification. The airlocks can be configured as air barrier, pressure-controlled or exclusively biological use, depending on the process requirements.
In addition, the isolator can be equipped with numerous optional accessories such as hooks on hanging bars, welders, cooling trays, cleaning accessories, etc.
An intuitive user interface allows all parameters (pressure, flow, humidity) to be controlled and alarms or anomalies to be displayed in real time.
Specific areas of application
Isolators have many applications in the pharmaceutical industry, mainly for production and control purposes. Isolators are useful when handling, transferring or packaging solid, semi-solid or powdered pharmaceutical products. They can also be used to handle and fill solutions and infusions. They enable sterility testing, defiltration, drying and weighing of cytotoxic substances. They are also used for the aseptic handling of tissues, biological production systems and pathogenic samples.
It also has a place in R&D for the formulation of biological products, in the manufacture of vaccines, and in the production of advanced therapies (ATMP).
What are the Advantages of Pharmaceutical Isolators?
A pharmaceutical isolator is a modern medical technology that has several advantages for the pharmaceutical industry. It offers a superior sterile environment compared to conventional environments such as sterile clean rooms. Positive or negative pressures inside the chamber prevent contamination of the operator or the external environment in case of abnormalities. It ensures long-lasting sterility in accordance with pharmaceutical regulations. It is particularly effective during aseptic processes, procedure validations, sterility tests, etc.
The various controls and monitoring are facilitated due to the integrated options and software. Setting and recording of temperatures, pressures, humidity levels, etc. are possible, as are leakage and sterility controls. The data collected will be useful during inspections by the regulatory authorities.
An isolator for the pharmaceutical industry reduces costs. The equipment takes up less space, reduces possible refurbishment costs. They are cheaper to maintain and service, and that means lower operating costs.
Jacomex can design and manufacture pharmaceutical isolator that perfectly meets your needs by opting for a custom-made equipment. The device, options and accessories delivered can match your exact process requirements.
Jacomex, your partner for an increasingly innovative industry
As a leader in isolation technology, JACOMEX can create isolators for you that comply with the most stringent requirements of the pharmaceutical and medical sectors. The G(iso) isolator, for example, is a piece of equipment specially designed for the pharmaceutical and medical sectors. It provides enhanced protection for products, the environment and operators under neutral gas in negative pressure. It is ideal for operations involving the handling of oxidisable or hygroscopic active ingredients, CMRs or toxic powders.
It has several possible applications: chemical synthesis and handling of active ingredients, OEB4 – OEB5 and cytotoxic products, manufacture of surgical implants, galenic R&D, quality control. Its design (surface treatment and roughness, rounded corners, etc.) is perfectly suited to address the challenges of isolators: ease of cleaning and optimised decontamination, safe filter replacement using bag-in-bag-out, controlled and confined waste management.
The device, options and accessories supplied comply with your process requirements.
Our team supports you from feasibility studies to qualification (IQ/OQ/PQ) and provides European after-sales service to ensure your compliance and operational performance.
With over 80 years of experience, Jacomex combines French expertise and an international network to meet your project needs worldwide.
By choosing a pharmaceutical isolator, you are opting for a high-performance, adaptable and economical containment solution. With Jacomex as your partner, you benefit from cutting-edge expertise to guarantee the quality, safety and compliance of your pharmaceutical processes.
For more information, please contact us. Our team of experts will respond promptly.